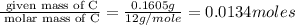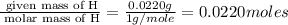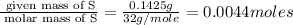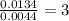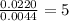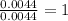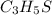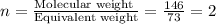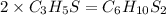Chemistry, 15.12.2020 15:50 constipatedcow18
A compound has a molecular weight of 146 g/mol. A 0.3250 g sample of the compound contains 0.1605 g of carbon, 0.0220 g of hydrogen, and 0.1425 g of sulfur. What is the molecular formula of the compound

Answers: 3
Another question on Chemistry



Chemistry, 21.06.2019 23:00
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1
You know the right answer?
A compound has a molecular weight of 146 g/mol. A 0.3250 g sample of the compound contains 0.1605 g...
Questions






Mathematics, 30.09.2020 02:01

Chemistry, 30.09.2020 02:01


Mathematics, 30.09.2020 02:01

History, 30.09.2020 02:01

Computers and Technology, 30.09.2020 02:01

Computers and Technology, 30.09.2020 02:01


Social Studies, 30.09.2020 02:01

Mathematics, 30.09.2020 02:01

Mathematics, 30.09.2020 02:01



Computers and Technology, 30.09.2020 02:01

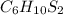
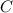 = 0.1605 g
= 0.1605 g
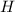 = 0.0220 g
= 0.0220 g
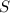 = 0.1425 g
= 0.1425 g