

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 23.06.2019 01:50
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2

Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2
You know the right answer?
A sample of helium gas at 841 mmHg and 14.7°C is heated to 84.7°C at constant volume. Calculate its...
Questions



Computers and Technology, 31.03.2021 02:30


Business, 31.03.2021 02:30


Chemistry, 31.03.2021 02:30

Biology, 31.03.2021 02:30


Biology, 31.03.2021 02:30

Mathematics, 31.03.2021 02:30

Mathematics, 31.03.2021 02:30


Mathematics, 31.03.2021 02:30

History, 31.03.2021 02:30


Mathematics, 31.03.2021 02:30

Mathematics, 31.03.2021 02:30


Mathematics, 31.03.2021 02:30

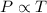 (At constant volume and number of moles)
(At constant volume and number of moles)

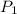 = initial pressure of gas = 841 mm Hg
= initial pressure of gas = 841 mm Hg
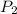 = final pressure of gas = ?
= final pressure of gas = ?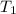 = initial temperature of gas =
= initial temperature of gas =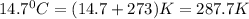
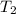 = final temperature of gas =
= final temperature of gas = 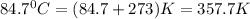
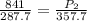
 ( 760 mm Hg = 1atm )
( 760 mm Hg = 1atm )

