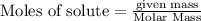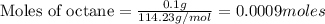Chemistry, 15.12.2020 16:40 BlueExorcistReaper
The enthalpy of combustion of octane is -5470 kJ/mol. Octane (formulae C8H18) was used to heat some water in a copper can. The amount of octane used up was 0.1 g. The amount of water in the can was 100 cm3 . a) Find the moles of octane which was used up. (Show calculations)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
The enthalpy of combustion of octane is -5470 kJ/mol. Octane (formulae C8H18) was used to heat some...
Questions

Mathematics, 04.02.2021 22:10

English, 04.02.2021 22:10

Advanced Placement (AP), 04.02.2021 22:10

Chemistry, 04.02.2021 22:10

Mathematics, 04.02.2021 22:10

Social Studies, 04.02.2021 22:10

Mathematics, 04.02.2021 22:10

Mathematics, 04.02.2021 22:10


Mathematics, 04.02.2021 22:10



Mathematics, 04.02.2021 22:10


English, 04.02.2021 22:10

Mathematics, 04.02.2021 22:10

Health, 04.02.2021 22:10

Mathematics, 04.02.2021 22:10


Chemistry, 04.02.2021 22:10