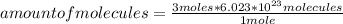Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3

Chemistry, 23.06.2019 08:50
Reacting masses1 calcium carbonate breaks down on heating to produce calcium oxide and carbondioxide gas.caco3 + cao + co2a student heats 15 g of calcium carbonate strongly in a crucible.relative atomic masses (a): ca = 40, c = 12, o = 16.calculate the mass of calcium oxide produced by this reaction.(5 marks)
Answers: 3
You know the right answer?
C3H5OH + 3 O2 --> CO2 + H2O When balancing the equation above, how many molecules of CO2 and H2O...
Questions

Mathematics, 29.09.2020 20:01


Mathematics, 29.09.2020 20:01



English, 29.09.2020 20:01



Mathematics, 29.09.2020 20:01



Mathematics, 29.09.2020 20:01






Mathematics, 29.09.2020 20:01

Mathematics, 29.09.2020 20:01