Worth a lot of points. Please only answer if u know the question. Thanks
...

Chemistry, 16.12.2020 01:10 fshane7705
Worth a lot of points. Please only answer if u know the question. Thanks
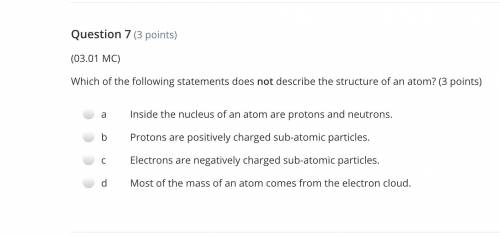

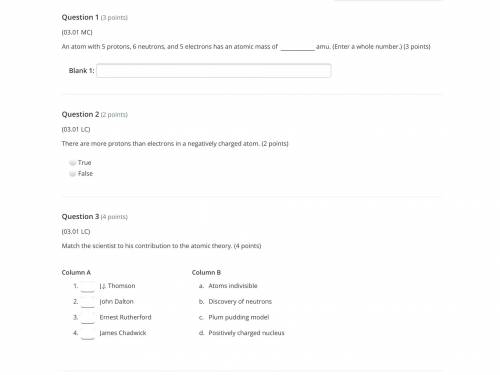

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 23.06.2019 17:20
1. list the number of drops of titrant needed to produce a color change for sample a and sample b. list the actual volume of titrant needed to produce a color change for sample a and sample b.
Answers: 1
You know the right answer?
Questions

History, 22.07.2019 03:20

Chemistry, 22.07.2019 03:20


Mathematics, 22.07.2019 03:20



Mathematics, 22.07.2019 03:20


Mathematics, 22.07.2019 03:20

Health, 22.07.2019 03:20

Health, 22.07.2019 03:20

Chemistry, 22.07.2019 03:20


History, 22.07.2019 03:20


Chemistry, 22.07.2019 03:20






