
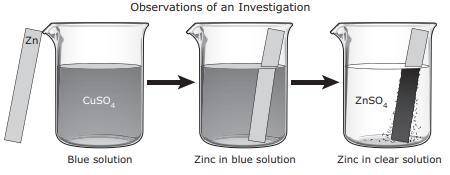
For an investigation a student poured a blue solution of CuSO4 into a beaker. The student placed a shiny, silver-colored strip of zinc metal in the solution and observed the changes. The student inferred that a chemical reaction occurred. What evidence supports this inference?
A. The CuSO4 solution turned blue when the zinc metal was added.
B. None of these
C. A dark solid formed on the zinc metal.
D. The zinc metal remained silver-colored and shiny.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1


Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
For an investigation a student poured a blue solution of CuSO4 into a beaker. The student placed a s...
Questions

Computers and Technology, 30.07.2021 09:50

Mathematics, 30.07.2021 09:50

Health, 30.07.2021 09:50

History, 30.07.2021 09:50




Business, 30.07.2021 09:50


English, 30.07.2021 09:50




English, 30.07.2021 09:50


English, 30.07.2021 09:50


English, 30.07.2021 09:50




