
Chemistry, 16.12.2020 05:40 stella013108
The balanced equation for the syntheis of Iron (I) oxide is below:
If 5.4 moles of Fe react with 4.7 moles of O2, what is the maximum amount of Fe2O3 (in moles) that can be produced? What is the limiting reactant?
a
3.1 moles of Fe2O3 is the maximum amount that can be produced. Oxygen is the limiting reactant.
b
2.7 moles of Fe2O3 is the maximum amount that can be produced. Iron is the limiting reactant.
c
7.1 moles of Fe2O3 is the maximum amount that can be produced. Oxygen is the limiting reactant.
d
10.8 moles of Fe2O3 is the maximum amount that can be produced. Iron is the limiting reactant.
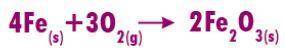

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
State the formula for density in words and mathematical symbols
Answers: 2

Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3


You know the right answer?
The balanced equation for the syntheis of Iron (I) oxide is below:
If 5.4 moles of Fe react with 4....
Questions


Chemistry, 22.01.2021 03:40



English, 22.01.2021 03:40




Advanced Placement (AP), 22.01.2021 03:40


English, 22.01.2021 03:40

Mathematics, 22.01.2021 03:40




Mathematics, 22.01.2021 03:40

Spanish, 22.01.2021 03:40





