
Chemistry, 16.12.2020 08:40 hgiaculliozdjov
Based on the reactivities of the elements involved, which reaction will form products that are more stable than the reactants?
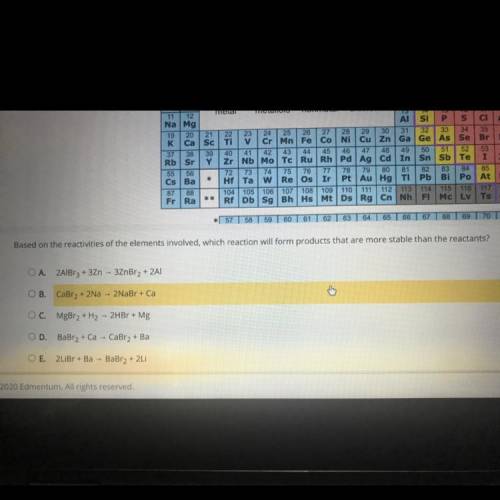

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:20
Amixture of gaseous sulfur dioxide and oxygen are added to a reaction vessel and heated to 1000 k where they react to form so3(g). if the vessel contains 0.669 atm so2(g), 0.395 atm o2(g), and 0.0851 atm so3(g) after the system has reached equilibrium, what is the equilibrium constant kp for the reaction: 2 so2(g) o2(g) ⇌ 2 so3(g)
Answers: 3

Chemistry, 21.06.2019 21:00
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1
You know the right answer?
Based on the reactivities of the elements involved, which reaction will form products that are more...
Questions

Mathematics, 30.11.2019 02:31

Mathematics, 30.11.2019 02:31

Mathematics, 30.11.2019 02:31



Mathematics, 30.11.2019 02:31

Mathematics, 30.11.2019 02:31

Mathematics, 30.11.2019 02:31


Physics, 30.11.2019 02:31


Mathematics, 30.11.2019 02:31




History, 30.11.2019 02:31

History, 30.11.2019 02:31






