
Chemistry, 16.12.2020 14:00 lesliealaves16
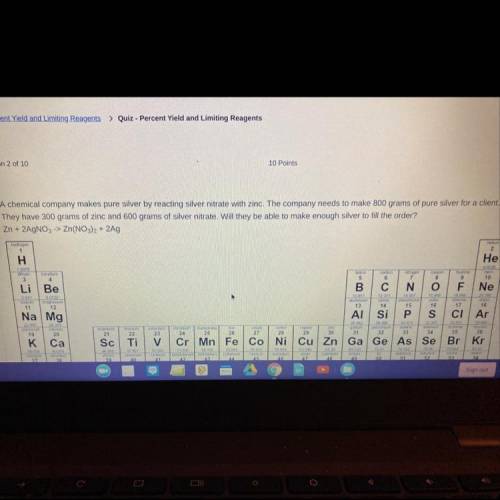
A chemical company makes pure silver by reacting silver nitrate with zinc. The company needs to make s00 grams of pure silver for a client.
They have 300 grams of zinc and 600 grams of silver nitrate. Will they be able to make enough silver to fil the order?
Zn + 2AgNO,> Zn(NO), + 2Ag A. Yes, there will be extra left over. B. Yes, they will have exactly enough C. No, the silver nitrate will run out first. D. No, the zinc nitrate will run out first

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
A chemical company makes pure silver by reacting silver nitrate with zinc. The company needs to make...
Questions

Mathematics, 20.05.2021 20:30




Mathematics, 20.05.2021 20:30




Mathematics, 20.05.2021 20:30





Mathematics, 20.05.2021 20:30


Advanced Placement (AP), 20.05.2021 20:30


English, 20.05.2021 20:30




