
Chemistry, 16.12.2020 16:40 5921000521
The mineral manganosite, manganese(ll) oxide, crystallizes in the rock salt structure the face-centered structure adopted by NaCl) with a density of 5.365 g/cm'. Find the unit cell edge length of manganosite.
A. 444.5 pm
B. 352.8 pm
C. 280.0 pm
D. 368.2 pm
E. 417.9 pm

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 22.06.2019 23:00
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
You know the right answer?
The mineral manganosite, manganese(ll) oxide, crystallizes in the rock salt structure the face-cente...
Questions

Biology, 07.12.2020 18:40

Biology, 07.12.2020 18:40

Mathematics, 07.12.2020 18:40

Mathematics, 07.12.2020 18:40

English, 07.12.2020 18:40

English, 07.12.2020 18:40

Advanced Placement (AP), 07.12.2020 18:40



Spanish, 07.12.2020 18:40


Mathematics, 07.12.2020 18:40


Chemistry, 07.12.2020 18:40

Social Studies, 07.12.2020 18:40



Mathematics, 07.12.2020 18:40


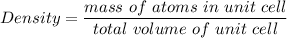
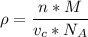
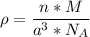
![[Mn(11)O] = 70.93 \ g/mol](/tpl/images/0990/1650/36da1.png)
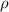 is given as 5.365 g/cm³
is given as 5.365 g/cm³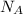 = 6.023 × 10²³ atoms/mol
= 6.023 × 10²³ atoms/mol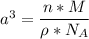
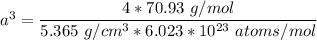
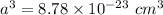
![a= \sqrt[3]{8.78 \times 10^{-23} \ cm^3}](/tpl/images/0990/1650/8dd66.png)


