
Chemistry, 16.12.2020 16:40 Redhead667
An aqueous solution of calcium hydroxide is standardized by titration with a 0.112 M solution of hydrobromic acid. If 15.2 mL of base are required to neutralize 12.4 mL of the acid, what is the molarity of the calcium hydroxide solution?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1



Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1
You know the right answer?
An aqueous solution of calcium hydroxide is standardized by titration with a 0.112 M solution of hyd...
Questions

Computers and Technology, 24.11.2021 02:50


Physics, 24.11.2021 02:50


History, 24.11.2021 02:50


Mathematics, 24.11.2021 02:50

Mathematics, 24.11.2021 02:50

English, 24.11.2021 02:50

Mathematics, 24.11.2021 02:50

Geography, 24.11.2021 02:50


Mathematics, 24.11.2021 02:50

Mathematics, 24.11.2021 02:50

History, 24.11.2021 02:50




History, 24.11.2021 02:50

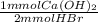 = 0.6944 mmol Ca(OH)₂
= 0.6944 mmol Ca(OH)₂


