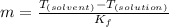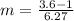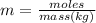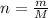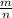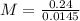Chemistry, 16.12.2020 17:00 trippyjazmine
When 240 mg of a certain molecular compound X are dissolved in 35.0 g of dibenzyl ether ((C6H5CH2)2O), the freezing point of the solution is measured to be 1.0 °C. Calculate the molar mass of X If you need any additional information on dibenzyl ether, use only what you find in the ALEKS Data resource. Also, be sure your answer has a unit symbol, and is rounded to the correct number of significant digits.

Answers: 1
Another question on Chemistry


Chemistry, 23.06.2019 03:30
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3

Chemistry, 23.06.2019 04:31
Which molecules are more strongly attracted to one another -c3h8o molecules that make up liquid rubbing alcohol or ch4 molecules that make up methane gas
Answers: 3

Chemistry, 23.06.2019 08:30
If you had to research a particular disease or area of concern in veterinary medicine and science, which one would you choose? why?
Answers: 1
You know the right answer?
When 240 mg of a certain molecular compound X are dissolved in 35.0 g of dibenzyl ether ((C6H5CH2)2O...
Questions

Mathematics, 22.01.2021 01:00

Biology, 22.01.2021 01:00

Mathematics, 22.01.2021 01:00





Health, 22.01.2021 01:00

Physics, 22.01.2021 01:00

Mathematics, 22.01.2021 01:00

Mathematics, 22.01.2021 01:00


English, 22.01.2021 01:00






Social Studies, 22.01.2021 01:00

Mathematics, 22.01.2021 01:00

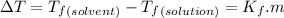
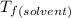 and
and 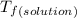 are freezing point of solvent and solution, respectively
are freezing point of solvent and solution, respectively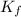 is freezing point depression constant
is freezing point depression constant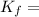 6.27 and
6.27 and 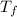 = 3.6°C.
= 3.6°C.