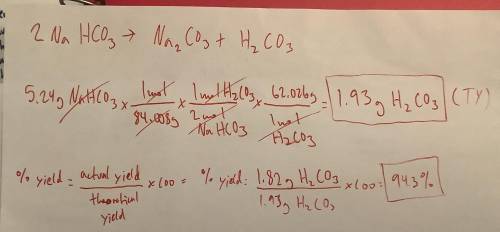Need an answer Asap
A 5.24-gram sample of NaHCO3 was completely decomposed in an experiment.
<...

Chemistry, 16.12.2020 18:20 jasminer257
Need an answer Asap
A 5.24-gram sample of NaHCO3 was completely decomposed in an experiment.
2NaHCO3 → Na2CO3 + H2CO3
In this experiment, carbon dioxide and water vapors combine to form H2CO3. After decomposition, the H2CO3 had a mass of 1.82 grams.
Determine the theoretical yield of the H2CO3 produced. Show your work.
Calculate the percentage yield of H2CO3 for the reaction. Show your work or describe the calculation process in detail.
(5 points)

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

You know the right answer?
Questions




Mathematics, 29.01.2020 06:51



Chemistry, 29.01.2020 06:51

Mathematics, 29.01.2020 06:51

English, 29.01.2020 06:51



English, 29.01.2020 06:51

History, 29.01.2020 06:51





Mathematics, 29.01.2020 06:51


History, 29.01.2020 06:51