
Chemistry, 16.12.2020 22:20 mgillis8816
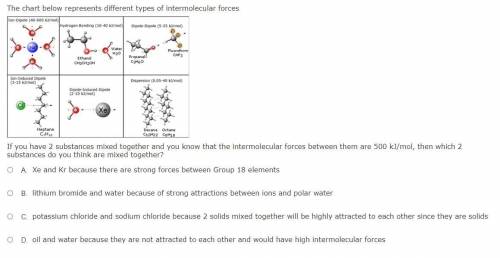
The chart below represents different types of intermolecular forces
If you have 2 substances mixed together and you know that the intermolecular forces between them are 500 kJ/mol, then which 2 substances do you think are mixed together?
A.
Xe and Kr because there are strong forces between Group 18 elements
B.
lithium bromide and water because of strong attractions between ions and polar water
C.
potassium chloride and sodium chloride because 2 solids mixed together will be highly attracted to each other since they are solids
D.
oil and water because they are not attracted to each other and would have high intermolecular forces

Answers: 1
Another question on Chemistry


Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1


Chemistry, 23.06.2019 07:30
Assume that 13.5 g solid aluminum (al) react with hcl to produce solid aluminum chloride (alcl3) salt and gaseous hydrogen (h2) at standard temperature and pressure.
Answers: 1
You know the right answer?
The chart below represents different types of intermolecular forces
If you have 2 substances mixed...
Questions



English, 04.10.2019 20:30

Biology, 04.10.2019 20:30




Mathematics, 04.10.2019 20:30



Mathematics, 04.10.2019 20:30

History, 04.10.2019 20:30


History, 04.10.2019 20:30

Mathematics, 04.10.2019 20:30




Physics, 04.10.2019 20:30



