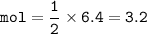Chemistry, 17.12.2020 02:40 leeney6166
Upon balancing the equation below, how many moles of sulfuric acid are needed to react completely with 6.4 moles of lithium hydroxide?
LiOH + H2SO4yields Li2SO4 + H2O (2 points)
Group of answer choices
12.8 mol H2SO4
6.4 mol H2SO4
3.2 mol H2SO4
2.1 mol H2SO4

Answers: 2
Another question on Chemistry


Chemistry, 21.06.2019 21:30
Which is the layer underground where all empty spaces are filled with a combination of air and water ?
Answers: 1

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

Chemistry, 23.06.2019 06:00
Which of the following is a solution a- brewed coffee b-tomato juice c- ranch salad dressing d- muddy water
Answers: 1
You know the right answer?
Upon balancing the equation below, how many moles of sulfuric acid are needed to react completely wi...
Questions


Physics, 24.04.2021 03:50

Social Studies, 24.04.2021 03:50


Mathematics, 24.04.2021 03:50



Health, 24.04.2021 03:50

Mathematics, 24.04.2021 03:50




Mathematics, 24.04.2021 03:50

Mathematics, 24.04.2021 03:50

Mathematics, 24.04.2021 03:50


Mathematics, 24.04.2021 03:50

Mathematics, 24.04.2021 03:50

Mathematics, 24.04.2021 03:50

Chemistry, 24.04.2021 03:50