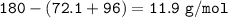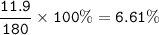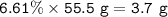Chemistry, 17.12.2020 03:00 azibur3191
Examination reveals that 180.2 g/mol of glucose contains 72.1 grams of carbon, 96 g/mol of oxygen and the remainder is hydrogen. How many g of hydrogen are there in 55.5 g of glucose?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2

Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2
You know the right answer?
Examination reveals that 180.2 g/mol of glucose contains 72.1 grams of carbon, 96 g/mol of oxygen an...
Questions





Mathematics, 05.10.2020 14:01



Mathematics, 05.10.2020 14:01

History, 05.10.2020 14:01

Biology, 05.10.2020 14:01

Mathematics, 05.10.2020 14:01


Mathematics, 05.10.2020 14:01

Social Studies, 05.10.2020 14:01

Mathematics, 05.10.2020 14:01

Mathematics, 05.10.2020 14:01

Biology, 05.10.2020 14:01



History, 05.10.2020 14:01