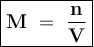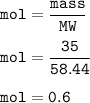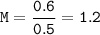Chemistry, 17.12.2020 03:10 babygirl10302015
A solution was prepared by dissolving 35.0 g of NaCl in water to make a 0.5 L solution. What is the molarity of the solution? The molar mass of NaCl = 58.44 g/mol.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
A solution was prepared by dissolving 35.0 g of NaCl in water to make a 0.5 L solution. What is the...
Questions


History, 07.10.2021 01:00


Biology, 07.10.2021 01:00

Computers and Technology, 07.10.2021 01:00


History, 07.10.2021 01:00


Mathematics, 07.10.2021 01:00

Mathematics, 07.10.2021 01:00

Mathematics, 07.10.2021 01:00


English, 07.10.2021 01:00




Mathematics, 07.10.2021 01:00

Computers and Technology, 07.10.2021 01:00