
Chemistry, 17.12.2020 05:20 vicky10445
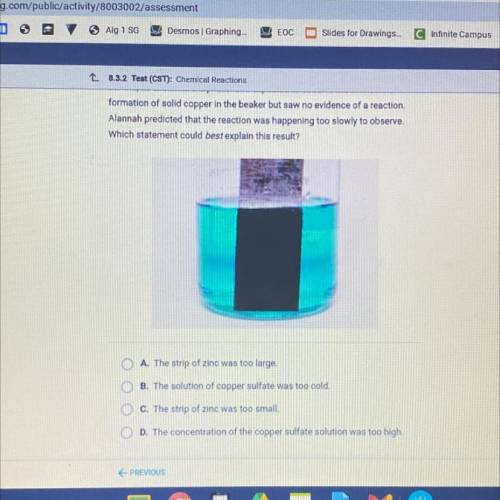
Alannah added a strip of zinc to a beaker containing a solution of copper
sulfate, as shown in the photo. She expected to observe the immediate
formation of solid copper in the beaker but saw no evidence of a reaction.
Alannah predicted that the reaction was happening too slowly to observe.
Which statement could best explain this result?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1


Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

You know the right answer?
Alannah added a strip of zinc to a beaker containing a solution of copper
sulfate, as shown in the...
Questions

Computers and Technology, 03.08.2021 04:10


Arts, 03.08.2021 04:10


Social Studies, 03.08.2021 04:10


Mathematics, 03.08.2021 04:10


Arts, 03.08.2021 04:10

English, 03.08.2021 04:10




English, 03.08.2021 04:10

History, 03.08.2021 04:10

Mathematics, 03.08.2021 04:10


Mathematics, 03.08.2021 04:10




