
Chemistry, 17.12.2020 08:10 ronniethefun
SOMEONE PLEASE HELP I DON'T GET THIS AT ALL
Modeling Energy Changes Student Guide on Edge
Step 3: Determine the amount of energy change in the reaction.
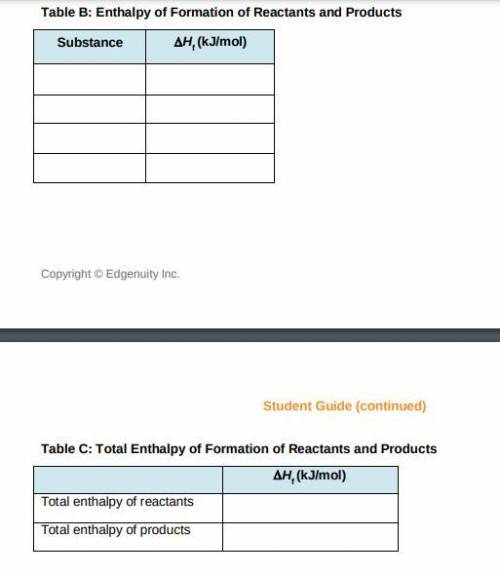
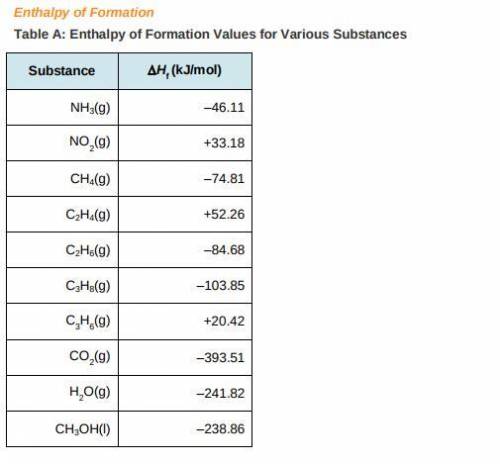
a) Use the table of enthalpy values (Table A) provided in the Student Worksheet to locate the enthalpy of formation (DeltaHt) for each reactant and each product. Record these values along with the reactants and products in Table B of the Student Worksheet.
b) Determine the total enthalpy of the reactants and the total enthalpy of the products Record these values in Table C of the Student Worksheet.
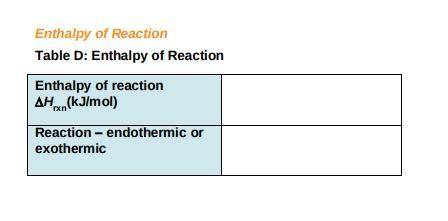
c) Use the following formula to find the net change in enthalpy for the reaction and to determine whether the reaction is endothermic is endothermic or exothermic.
ΔHrxn= ∑ (Δ Hf, products)- ∑ (ΔHf, reactants)
Record your answers in Table D.
Step 4: Model the energy change in the reaction.
a) Create an energy graph that illustrates the energy change in the reaction.
b)Construct your graph on a blank sheet of paper. Be sure to label the axes, provide a title, and identify the reactants and product on the graph.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

You know the right answer?
SOMEONE PLEASE HELP I DON'T GET THIS AT ALL
Modeling Energy Changes Student Guide on Edge
Questions

Mathematics, 16.07.2019 21:30


Mathematics, 16.07.2019 21:30

History, 16.07.2019 21:30

Mathematics, 16.07.2019 21:30

Health, 16.07.2019 21:30



Mathematics, 16.07.2019 21:30




Mathematics, 16.07.2019 21:30



History, 16.07.2019 21:30

Mathematics, 16.07.2019 21:30


Mathematics, 16.07.2019 21:30

Mathematics, 16.07.2019 21:30



