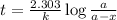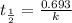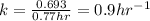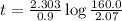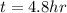Chemistry, 17.12.2020 18:00 samanthabutryn
The half-life of the radioisotope 158Eu is 0.77 h. How much time is required for a 160.0-g sample of 158Eu to decay to 2.07 g?
a. 3.0 h
b. 4.0 h
c. 6.0 h
d. 2.1 h
e. 4.8 h

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3


Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3
You know the right answer?
The half-life of the radioisotope 158Eu is 0.77 h. How much time is required for a 160.0-g sample of...
Questions

Chemistry, 14.10.2019 10:30




Mathematics, 14.10.2019 10:30




History, 14.10.2019 10:30

Mathematics, 14.10.2019 10:30

History, 14.10.2019 10:30





Physics, 14.10.2019 10:30