
Chemistry, 17.12.2020 19:00 erinolson07cats
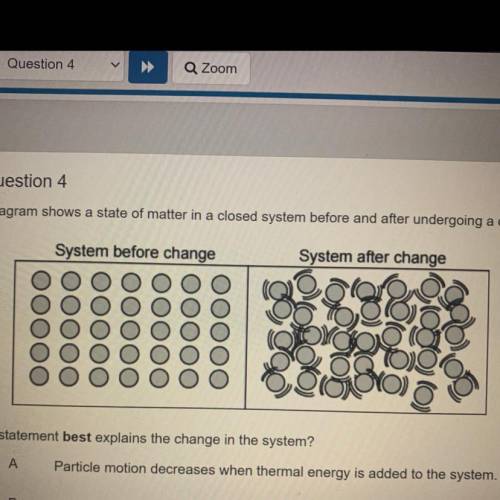
The diagram shows a state of matter in a closed system before and after undergoing a change.
System before change
System after change
Svo
Which statement best explains the change in the system?
Particle motion decreases when thermal energy is added to the system.
A
B
Particle motion increases when solid particles are added to the system.
С
Particle motion increases when thermal energy is added to the system.
D
Particle motion decreases when gas particles are added to the system.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 23.06.2019 06:30
Which of these describes how heat is transferred by convection* a. sunlight travels through space without the aid of fluids or solids. b. warm air rises and takes the heat with it, eventually, it cools and sinks c. air at the equator rises and sinks at the poles. d. air molecules touch the warm ground, heating them up *not conduction
Answers: 3

Chemistry, 23.06.2019 14:00
During an acid-base titration, when do the contents of the beaker consist of only water, a salt, and a trace of indicator?
Answers: 2

Chemistry, 23.06.2019 15:30
Select the correct answer.the gas in a sealed container has an absolute pressure of 125.4 kilopascals. if the air around the container is at a pressure of 99.8 kilopascals, what is thegauge pressure inside the container?
Answers: 3
You know the right answer?
The diagram shows a state of matter in a closed system before and after undergoing a change.
System...
Questions















Mathematics, 11.02.2020 17:19







