
Chemistry, 17.12.2020 20:00 mikurrjurdan
The synthesis of NH3 is represented by the equation above. based on the equilibrium constant, K and delta H rxn given above, which of the following can best be used to justify that the reaction is thermodynamically favorable at 298 K and constant pressure?
N2 (g) + 3H2 (g) --> 2NH3 (g)
K= 5.6 × 10^5 at 298 K
delta H rxn= -91.8 kj/mol
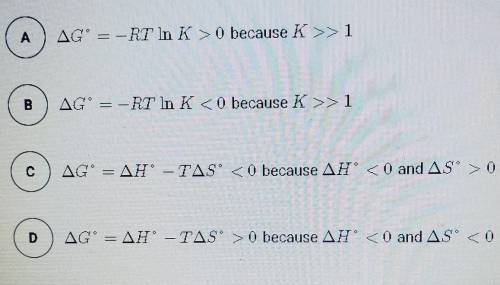

Answers: 3
Another question on Chemistry


Chemistry, 21.06.2019 23:30
Agroup of students is studying convection currents. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other in an area with warm air. after 10 minutes, the balloons are released from a height of 1 meter. which of the following do the students most likely observe? a. the balloons both rise. the cold balloon is larger than the warm balloon. b. the balloons rise at the same rate. both balloons are the same size. c. the warm balloon expands and rises. the cold balloon shrinks and sinks. d. the cold balloon expands and rises. the warm balloon shrinks and sinks.
Answers: 2

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
The synthesis of NH3 is represented by the equation above. based on the equilibrium constant, K and...
Questions





English, 04.09.2019 22:30




Mathematics, 04.09.2019 22:30

Mathematics, 04.09.2019 22:30



English, 04.09.2019 22:30




Mathematics, 04.09.2019 22:30

Mathematics, 04.09.2019 22:30

History, 04.09.2019 22:30

English, 04.09.2019 22:30



