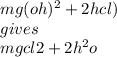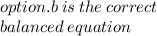Chemistry, 17.12.2020 20:40 nessuhbae6731
What is the complete balanced equation for the reaction
between magnesium hydroxide and hydrogen chloride to
produce magnesium chloride and water is
O MgOH + HCI → MgCl + H2O.
O Mg(OH)2 + 2HCl → MgCl2 + 2H20.
O Mg(OH)2 + HCl → Mg + 2CO2 +3H20.
O Mg(OH)2 + 2HCl → MgCl2 + H2 + O2.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3


Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1
You know the right answer?
What is the complete balanced equation for the reaction
between magnesium hydroxide and hydrogen ch...
Questions

Mathematics, 22.01.2021 14:00

Mathematics, 22.01.2021 14:00


Geography, 22.01.2021 14:00

Arts, 22.01.2021 14:00

English, 22.01.2021 14:00

Chemistry, 22.01.2021 14:00


English, 22.01.2021 14:00

History, 22.01.2021 14:00





History, 22.01.2021 14:00

Mathematics, 22.01.2021 14:00

Mathematics, 22.01.2021 14:00

Mathematics, 22.01.2021 14:00