
Chemistry, 18.12.2020 01:00 reneebrown017
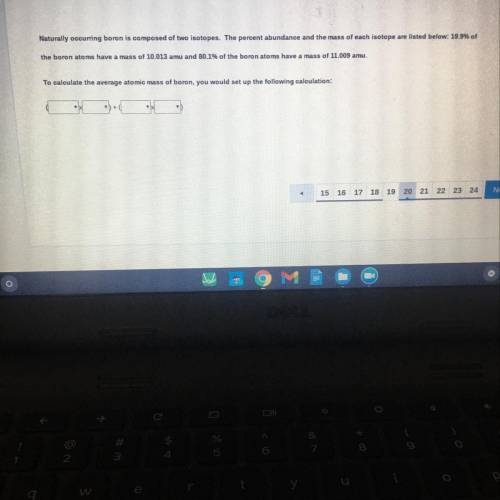
Naturally occurring boron is composed of two isotopes. The percent abundance and the mass of each isotope are listed below: 19.9% of
ne boron atoms have a mass of 10.013 amu and 80.1% of the boron atoms have a mass of 11.009 amu.
calculate the average atomic mass of boron, you would set up the following calculation:
What

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5 m hcl? show all of the work needed to solve this problem. mg (s) + 2hcl (aq) → mgcl2 (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3
You know the right answer?
Naturally occurring boron is composed of two isotopes. The percent abundance and the mass of each is...
Questions



English, 27.10.2020 18:20

Mathematics, 27.10.2020 18:20


Social Studies, 27.10.2020 18:20

Mathematics, 27.10.2020 18:20

English, 27.10.2020 18:20

Geography, 27.10.2020 18:20

Mathematics, 27.10.2020 18:20

Law, 27.10.2020 18:20

History, 27.10.2020 18:20

Mathematics, 27.10.2020 18:20



Mathematics, 27.10.2020 18:20


Mathematics, 27.10.2020 18:20

Mathematics, 27.10.2020 18:20

Mathematics, 27.10.2020 18:20



