
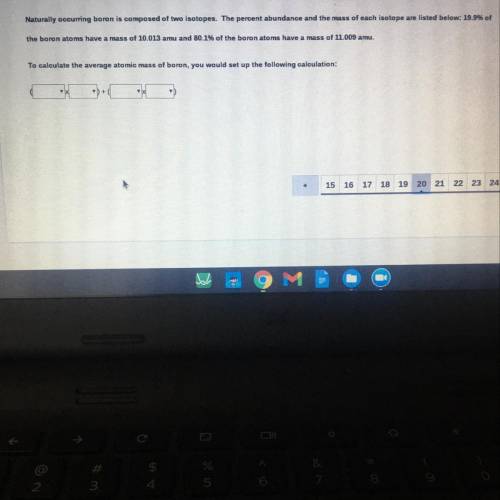
Naturally occurring boron is composed of two isotopes. The percent abundance and the mass of each isotope are listed below: 19.99
the boron atoms have a mass of 10.013 amu and 80.1% of the boron atoms have a mass of 11.009 amu.
To calculate the average atomic mass of boron, you would set up the following calculation:
+

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
You know the right answer?
Naturally occurring boron is composed of two isotopes. The percent abundance and the mass of each is...
Questions


Physics, 12.07.2019 21:00

Mathematics, 12.07.2019 21:00






History, 12.07.2019 21:00

Mathematics, 12.07.2019 21:00

History, 12.07.2019 21:00

History, 12.07.2019 21:00

Business, 12.07.2019 21:00

Mathematics, 12.07.2019 21:00





Social Studies, 12.07.2019 21:00



