
Chemistry, 18.12.2020 04:10 eylinglez3ovm16v
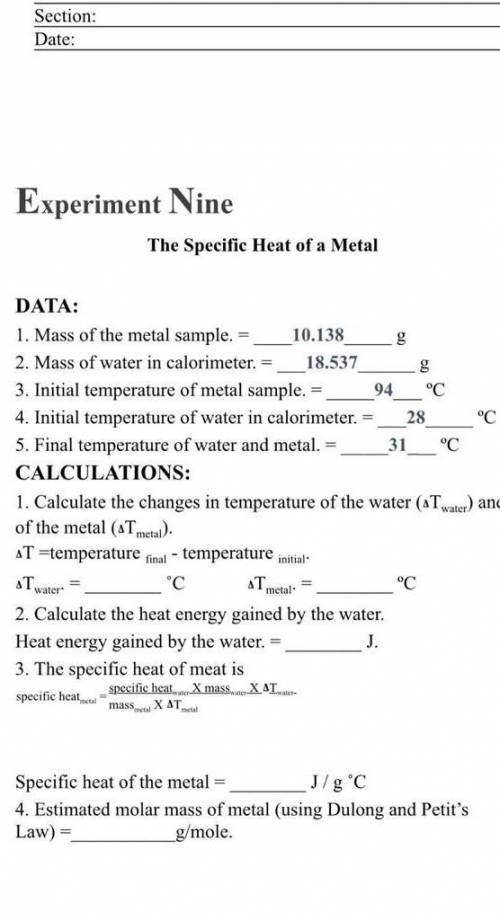
Experiment Nine
The Specific Heat of a Metal
DATA:
1. Mass of the metal sample.
10.138
2. Mass of water in calorimeter. 18.537
3. Initial temperature of metal sample. 94 °C
4. Initial temperature of water in calorimeter. 28 °C
5. Final temperature of water and metal. 31___"C
CALCULATIONS:
1. Calculate the changes in temperature of the water (aT water) and
of the metal (ATmetal).
T =temperature final - temperature initial
"С ST
°C
2. Calculate the heat energy gained by the water.
Heat energy gained by the water. =
J.
3. The specific heat of meat is
specific heat mass. XAT.
specific heal
Tnator
metal
mask.
X ΔΤ.
Specific heat of the metal
J/g °C
4. Estimated molar mass of metal (using Dulong and Petit's
Law) =
g/mole.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2
You know the right answer?
Experiment Nine
The Specific Heat of a Metal
DATA:
1. Mass of the metal sample.
10.1...
DATA:
1. Mass of the metal sample.
10.1...
Questions

Mathematics, 27.01.2021 21:00

Biology, 27.01.2021 21:00

Computers and Technology, 27.01.2021 21:00


Chemistry, 27.01.2021 21:00


Mathematics, 27.01.2021 21:00

Computers and Technology, 27.01.2021 21:00


Mathematics, 27.01.2021 21:00



History, 27.01.2021 21:00


History, 27.01.2021 21:00



Mathematics, 27.01.2021 21:00


Advanced Placement (AP), 27.01.2021 21:00




