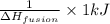Chemistry, 18.12.2020 09:50 Goldenstate32
How is the AHfusion used to calculate the mass of solid that 1kJ of energy will melt? O A. 1kJ * AHfusion A Hfusion x g/mol solid B. 1kJ x 1/ AHfusion * g/mol solid O C. 1kJ x 1/A Hfusion * mol/g solid D. 1kJ * AHfusion x mol/g solid

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1
You know the right answer?
How is the AHfusion used to calculate the mass of solid that 1kJ of energy will melt? O A. 1kJ * AHf...
Questions

Mathematics, 21.06.2021 16:50



Health, 21.06.2021 16:50






Computers and Technology, 21.06.2021 16:50


Mathematics, 21.06.2021 16:50

Biology, 21.06.2021 16:50

Mathematics, 21.06.2021 16:50


Mathematics, 21.06.2021 16:50




History, 21.06.2021 16:50

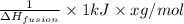
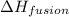 is used to melt = 1 mole of solid
is used to melt = 1 mole of solid