
Chemistry, 18.12.2020 18:30 kailahgranger
Question 5 (1 point)
Based on the Lewis diagrams of the compounds below, predict which compound has the strongest bonds.
A.
B.
C.
D.
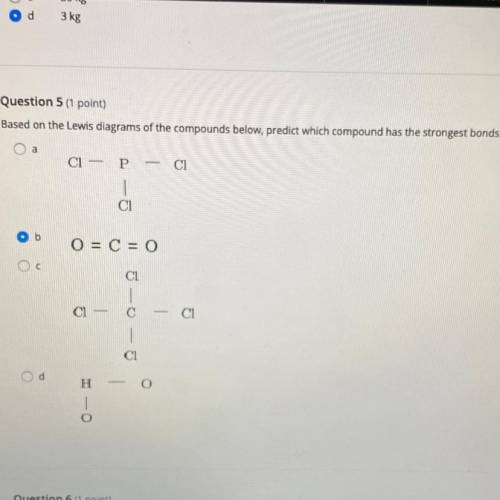

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1
You know the right answer?
Question 5 (1 point)
Based on the Lewis diagrams of the compounds below, predict which compound has...
Questions



Health, 03.02.2020 04:50

English, 03.02.2020 04:50





Mathematics, 03.02.2020 04:50



English, 03.02.2020 04:50

Mathematics, 03.02.2020 04:50


Mathematics, 03.02.2020 04:50


Social Studies, 03.02.2020 04:50

History, 03.02.2020 04:50


Mathematics, 03.02.2020 04:50



