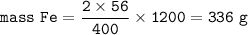Chemistry, 19.12.2020 18:20 brainguy124
Cuántos gramos de hierro están contenidos en una masa de sulfato férrico, de manera que esta última sustancia contenga 3/5 del número de moléculas que contiene 220 gramos de CO2? P. A. (uma) : Fe = 56 ; S = 32 ; O = 16 ; C = 12

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 12:30
17) large amounts of very important metal titanium are made by reacting magnesium metal with titanium tetrachloride. titanium metal and magnesium chloride are produced. a) write the balanced equation for this reaction. b) how many kilograms of magnesium are required to produce 1.00 kilograms of titanium? ( show work, .)
Answers: 1
You know the right answer?
Cuántos gramos de hierro están contenidos en una masa de sulfato férrico, de manera que esta última...
Questions

Computers and Technology, 22.02.2021 16:00

Mathematics, 22.02.2021 16:00

English, 22.02.2021 16:00




Mathematics, 22.02.2021 16:00


Mathematics, 22.02.2021 16:00

Mathematics, 22.02.2021 16:00

English, 22.02.2021 16:00







English, 22.02.2021 16:10

Biology, 22.02.2021 16:10

English, 22.02.2021 16:10

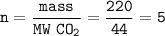
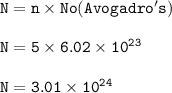
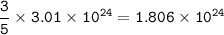
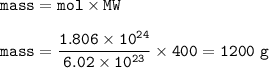 :
: