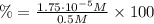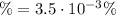Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3
You know the right answer?
Ka/KbMIXED PRACTICE108.Calculate the [H3O+(aq)], the pH, and the % reaction for a 0.50 mol/L HCN sol...
Questions

Social Studies, 26.08.2019 11:10

History, 26.08.2019 11:10


Physics, 26.08.2019 11:10

Mathematics, 26.08.2019 11:10


English, 26.08.2019 11:10

Mathematics, 26.08.2019 11:10



Mathematics, 26.08.2019 11:10

Mathematics, 26.08.2019 11:10

Social Studies, 26.08.2019 11:10

Biology, 26.08.2019 11:10

Mathematics, 26.08.2019 11:10

History, 26.08.2019 11:10

Geography, 26.08.2019 11:10

History, 26.08.2019 11:10


![Ka = \frac{[H_{3}O^{+}][CN^{-}]}{[HCN]} = 6.17 \cdot 10^{-10}](/tpl/images/1003/6190/82ff7.png)
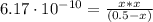
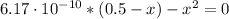
![pH = -log[H_{3}O^{+}] = -log(1.75 \cdot 10^{-5} M) = 4.75](/tpl/images/1003/6190/329a3.png)
![\% = \frac{x}{[HCN]} \times 100](/tpl/images/1003/6190/4a3ff.png)