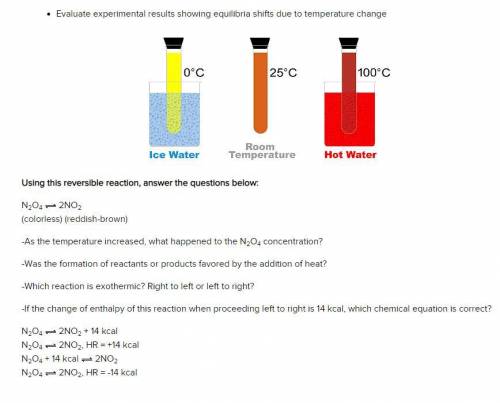
Using this reversible reaction, answer the questions below:
N2O4 2NO2
(colorless) (reddish-br...

Chemistry, 21.12.2020 14:00 mommer2019
Using this reversible reaction, answer the questions below:
N2O4 2NO2
(colorless) (reddish-brown)
-As the temperature increased, what happened to the N2O4 concentration?
-Was the formation of reactants or products favored by the addition of heat?
-Which reaction is exothermic? Right to left or left to right?
-If the change of enthalpy of this reaction when proceeding left to right is 14 kcal, which chemical equation is correct?
N2O4 2NO2 + 14 kcal
N2O4 2NO2, HR = +14 kcal
N2O4 + 14 kcal 2NO2
N2O4 2NO2, HR = -14 kcal

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1

Chemistry, 23.06.2019 06:00
What physical property of gold makes panning a useful way to get gold from streams?
Answers: 2
You know the right answer?
Questions



Mathematics, 08.12.2021 14:00

Mathematics, 08.12.2021 14:00

English, 08.12.2021 14:00

Mathematics, 08.12.2021 14:00



Mathematics, 08.12.2021 14:00


Mathematics, 08.12.2021 14:00

Computers and Technology, 08.12.2021 14:00



Mathematics, 08.12.2021 14:00

German, 08.12.2021 14:00


Mathematics, 08.12.2021 14:00

English, 08.12.2021 14:00

Mathematics, 08.12.2021 14:00




