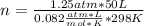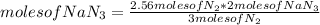Chemistry, 21.12.2020 17:40 deandrebryant89
Automobile air bags inflate during a crash or sudden stop by the rapid generation of nitrogen gas from sodium azide. 2NaN3(s) -->2Na(s) + 3N2 (g)How many moles of sodium azide are needed to produce sufficient nitrogen to fill a 50.0 L air bag to a pressure of 1.25 atm at 25C?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 12:30
Infants born with severe respiratory problems are sometimes given liquid ventilation: they breathe a liquid that can dissolve more oxygen than air can hold. one of these liquids is a fluorinated compound, cf3(cf2)7br. the solubility of oxygen in this liquid is 66 mlo2 per 100 ml liquid. in contrast, air is 21 % oxygen by volume. calculate the moles of o2 present in an infant's lungs (volume: 12 ml ) if the infant takes a full breath of air. assume a pressure of 1 atm in the lungs.
Answers: 1

Chemistry, 21.06.2019 18:00
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2
You know the right answer?
Automobile air bags inflate during a crash or sudden stop by the rapid generation of nitrogen gas fr...
Questions

Mathematics, 29.01.2021 21:50


Mathematics, 29.01.2021 21:50

Mathematics, 29.01.2021 21:50


Mathematics, 29.01.2021 21:50

Spanish, 29.01.2021 21:50

Mathematics, 29.01.2021 21:50



History, 29.01.2021 21:50

Mathematics, 29.01.2021 21:50

Mathematics, 29.01.2021 21:50

World Languages, 29.01.2021 21:50

Mathematics, 29.01.2021 21:50


Biology, 29.01.2021 21:50



Mathematics, 29.01.2021 21:50

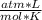 T= 25 C= 298 K
T= 25 C= 298 K