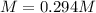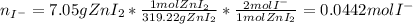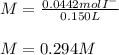Chemistry, 21.12.2020 17:30 aidenbender06
Suppose 7.05 g of zinc iodide is dissolved in 150. mL of a 0.20M aqueous solution of potassium carbonate. Calculate the final molarity of iodide anion in the solution. You can assume the volume of the solution doesn't change when the zinc iodide is dissolved in it. Round your answer to 3 significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2


Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1
You know the right answer?
Suppose 7.05 g of zinc iodide is dissolved in 150. mL of a 0.20M aqueous solution of potassium carbo...
Questions

Mathematics, 24.03.2021 06:40

Mathematics, 24.03.2021 06:40


English, 24.03.2021 06:40

Social Studies, 24.03.2021 06:40

Mathematics, 24.03.2021 06:40

Mathematics, 24.03.2021 06:40

Mathematics, 24.03.2021 06:40



Biology, 24.03.2021 06:40


Mathematics, 24.03.2021 06:40


Mathematics, 24.03.2021 06:40

Mathematics, 24.03.2021 06:40

Computers and Technology, 24.03.2021 06:40

Mathematics, 24.03.2021 06:40

Mathematics, 24.03.2021 06:40

Mathematics, 24.03.2021 06:40