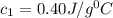When 100.0 g block of a metal at 600 oC is plunged into 100.0 g of water (specific heat capacity 4.2 J/g. oC) at 30 oC, the final temperature of both the metal and the water is 80 oC. If no heat is lost to the surroundings, what is the specific heat capacity of the metal in J/g. oC? Give your answer to 2 significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 23.06.2019 08:00
Ineed this awnser fast select the correct answer. this chemical equation represents the burning of methane, but the equation is incomplete. what is the missing coefficient in both the reactants and the products? ch4 + → co2 + a. 0 b. 1c. 2d. 3 e. 4
Answers: 3

Chemistry, 23.06.2019 13:00
Sort these isotopes by whether they are most likely to undergo fusion or fission. hydrogen-3, uranium-233, plutonium-239, hydrogen-1, helium-3, plutonium-241
Answers: 2

Chemistry, 23.06.2019 14:20
What kind of chemical reaction does the chemical equation sodium + chlorine → sodium chloride represent? a. combustion b. decomposition c. single replacement d. synthesis
Answers: 1
You know the right answer?
When 100.0 g block of a metal at 600 oC is plunged into 100.0 g of water (specific heat capacity 4.2...
Questions

Physics, 29.08.2021 04:40

Mathematics, 29.08.2021 04:40

Biology, 29.08.2021 04:40

Mathematics, 29.08.2021 04:40


Mathematics, 29.08.2021 04:40

Mathematics, 29.08.2021 04:40

History, 29.08.2021 04:40

Chemistry, 29.08.2021 04:40

Mathematics, 29.08.2021 04:40

Biology, 29.08.2021 04:40

Biology, 29.08.2021 04:40

Mathematics, 29.08.2021 04:40


Mathematics, 29.08.2021 04:40

English, 29.08.2021 04:40


Mathematics, 29.08.2021 04:40


Mathematics, 29.08.2021 04:50


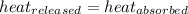

![-[m_1\times c_1\times (T_{final}-T_1)]=[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/1004/1883/92a72.png) .................(1)
.................(1)
 = mass of metal = 100.0 g
= mass of metal = 100.0 g
 = mass of water = 100.0 g
= mass of water = 100.0 g
 = final temperature =
= final temperature = 
 = temperature of metal =
= temperature of metal = 
 = temperature of water =
= temperature of water = 
 = specific heat of metal = ?
= specific heat of metal = ? = specific heat of water=
= specific heat of water= 
![-[100.0\times c_1\times (80-600)]=[100.0\times 4.2\times (80-30)]](/tpl/images/1004/1883/4e506.png)