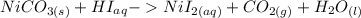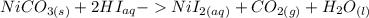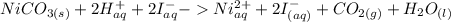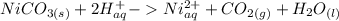Chemistry, 22.12.2020 04:40 vanessacasillas452
The net ionic equation for formation of an aqueous solution of NiI2 accompanied by evolution of CO2 gas via mixing solid NiCO3 and aqueous hydriodic acid is .A) 2NiCO3 (s) + HI (aq) ® 2H2O (l) + CO2 (g) + 2Ni2+ (aq)B) NiCO3 (s) + I- (aq) ® 2H2O (l) + CO2 (g) + Ni2+ (aq) + HI (aq)C) NiCO3 (s) + 2H+ (aq) ® H2O (l) + CO2 (g) + Ni2+ (aq)D) NiCO3 (s) + 2HI (aq) ® 2H2O (l) + CO2 (g) + NiI2 (aq)E) NiCO3 (s) + 2HI (aq) ® H2O (l) + CO2 (g) + Ni2+ (aq) + 2I- (aq)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:40
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3
You know the right answer?
The net ionic equation for formation of an aqueous solution of NiI2 accompanied by evolution of CO2...
Questions



History, 12.04.2021 01:00


History, 12.04.2021 01:00


History, 12.04.2021 01:00

Social Studies, 12.04.2021 01:00



Mathematics, 12.04.2021 01:00


World Languages, 12.04.2021 01:00


Mathematics, 12.04.2021 01:00


Mathematics, 12.04.2021 01:00



Mathematics, 12.04.2021 01:00