
Chemistry, 22.12.2020 06:40 91miketaylor
Two isotopes of nickel have a percent abundance of 0.93% and 68.08% with masses of 63.93
amu and 57.93 amu respectively. What is the atomic mass of these two isotopes? Explain why
the average mass is closer to actual amu of 58.69 for nickel.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1


You know the right answer?
Two isotopes of nickel have a percent abundance of 0.93% and 68.08% with masses of 63.93
amu and 57...
Questions

Mathematics, 11.12.2019 20:31




Mathematics, 11.12.2019 20:31




English, 11.12.2019 20:31


Mathematics, 11.12.2019 20:31


Biology, 11.12.2019 20:31

Business, 11.12.2019 20:31



Mathematics, 11.12.2019 20:31


Social Studies, 11.12.2019 20:31

English, 11.12.2019 20:31



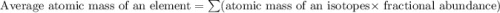
![A=\sum[(63.93)\times \frac{0.93}{100})+(57.93)\times \frac{68.08}{100}]]](/tpl/images/1005/1681/01772.png)



