
Chemistry, 22.12.2020 08:40 anaroles04
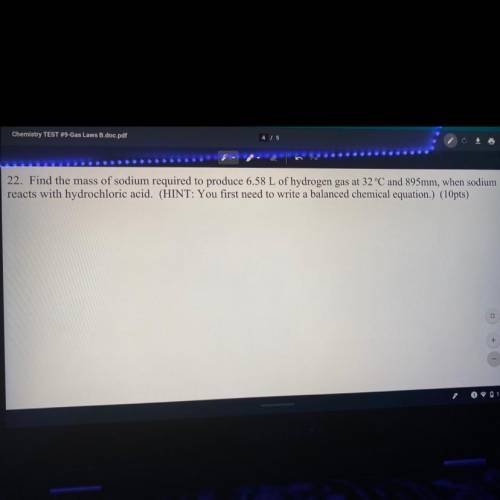
Find the mass of sodium required to reduce 6.58 L of hydrogen gas at 32°C and 895 mm, when sodium reacts with hydrochloric acid. (Hint: you first need to write a balanced chemical equation.)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
Find the mass of sodium required to reduce 6.58 L of hydrogen gas at 32°C and 895 mm, when sodium re...
Questions


English, 09.04.2020 22:55


Mathematics, 09.04.2020 22:55



Mathematics, 09.04.2020 22:55




Mathematics, 09.04.2020 22:56


Mathematics, 09.04.2020 22:56



Mathematics, 09.04.2020 22:56



Mathematics, 09.04.2020 22:56

Mathematics, 09.04.2020 22:56



