
Chemistry, 22.12.2020 17:00 tommylopez22713
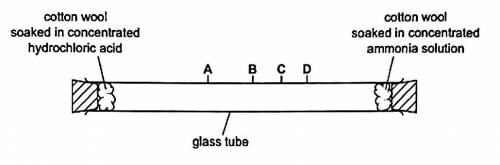
Concentrated ammonia solution gives off ammonia gas. Concentrated hydrochloric acid gives off hydrogen chloride gas. Ammonia, NH³ and hydrogen chloride, HCI , are both colourless gases. ammonia reacts with hydrogen chloride to make the white solid ammonium chloride 1.at which point A, B,C or D does the white solid form? Explain why the white solid forms at that point

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 23.06.2019 07:00
What is the difference between covalent bonds and ionic bonds? covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between charged atoms. covalent bonds involve the transfer of electrons between charged atoms; ionic bonds involve the sharing of electrons between atoms. covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms. covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between charged atoms.
Answers: 1
You know the right answer?
Concentrated ammonia solution gives off ammonia gas. Concentrated hydrochloric acid gives off hydrog...
Questions


Physics, 28.12.2019 17:31




Mathematics, 28.12.2019 17:31









Mathematics, 28.12.2019 17:31

Chemistry, 28.12.2019 17:31

Biology, 28.12.2019 17:31

Mathematics, 28.12.2019 17:31


Advanced Placement (AP), 28.12.2019 17:31



