
Chemistry, 22.12.2020 19:00 ShawnSaviro4918
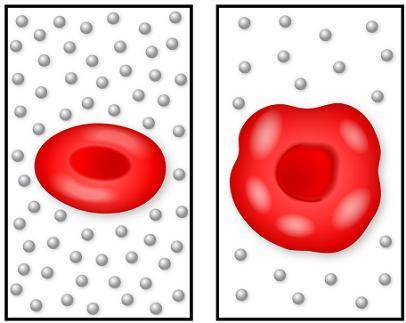
The image on the left shows a normal red blood cell, and the image on the right shows a cell that has been put into a new solution. The circles represent the salt in the solutions.
Which statement describes the motion of the water molecules in this situation?
The water molecules move by active transport into the cell from low water concentration to high water concentration.
The water molecules move by osmosis into the cell from low water concentration to high water concentration.
The water molecules move by osmosis into the cell from high water concentration to low water concentration.
The water molecules move by active transport into the cell from high water concentration to low water concentration.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

You know the right answer?
The image on the left shows a normal red blood cell, and the image on the right shows a cell that ha...
Questions

Mathematics, 03.12.2021 20:20




Social Studies, 03.12.2021 20:20


Mathematics, 03.12.2021 20:20


Mathematics, 03.12.2021 20:20

Computers and Technology, 03.12.2021 20:20

Mathematics, 03.12.2021 20:20

Computers and Technology, 03.12.2021 20:20


Biology, 03.12.2021 20:20

English, 03.12.2021 20:20

English, 03.12.2021 20:20




Spanish, 03.12.2021 20:20



