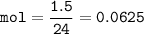Chemistry, 22.12.2020 19:10 Flowershere121
Sodium hydrogen carbonate, on heating, produces sodium carbonate, water and carbon dioxide.
A recipe for chocolate chip cookies requires 1.5 dm' of carbon dioxide.
Calculate the mass of sodium hydrogen carbonate that should be used at R. T.P.
INa = 23; H = 1: C = 12; O = 16.]
[Note that 1 mole of gas occupies a volume of 24,000 cm at room temperature and pressure (RTP)]

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
What is the theoretical yield of carbon dioxide? a)0.993 gb)2.98 gc)3.65 gd)8.93 g
Answers: 1

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2
You know the right answer?
Sodium hydrogen carbonate, on heating, produces sodium carbonate, water and carbon dioxide.
A recip...
Questions


Mathematics, 03.03.2021 23:40

Chemistry, 03.03.2021 23:40

Mathematics, 03.03.2021 23:40


English, 03.03.2021 23:40

History, 03.03.2021 23:40


Law, 03.03.2021 23:40


Mathematics, 03.03.2021 23:40

Mathematics, 03.03.2021 23:40


Biology, 03.03.2021 23:50

Mathematics, 03.03.2021 23:50


Mathematics, 03.03.2021 23:50


History, 03.03.2021 23:50

Mathematics, 03.03.2021 23:50