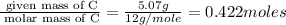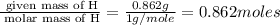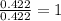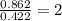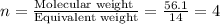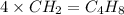Chemistry, 23.12.2020 01:00 kcameronanderso
When 6.040 grams of a hydrocarbon, CxHy, were burned in a combustion analysis apparatus, 18.95 grams of CO2 and 7.759 grams of H2O were produced. In a separate experiment, the molar mass of the compound was found to be 56.11 g/mol. Determine the empirical formula and the molecular formula of the hydrocarbon. Enter the elements in the order presented in the question.

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 07:00
Under what conditions will a gas be most likely to exhibit the ideal gas properties predicted by the ideal gas law? 1)high pressures and high temperature, because particles are forced closer together with higher kinetic energy, so intermolecular forces of attraction are weaker 2)high pressure and low temperature, because particles are forced closer together and moving slower, so the volume of the particles is less significant 3) low pressure and high temperature, because particles are spread farther apart and moving faster, so the intermolecular forces of attraction are weaker 4)low pressure and low temperature, because particles are spread farther apart with lower kinetic energy, so the volume of the particles is less significant
Answers: 2

Chemistry, 23.06.2019 10:00
Which element forms a compound with chlorine with the general formula mci?
Answers: 1

Chemistry, 23.06.2019 23:00
Putting maximum amount of points a solution is made by dissolving 10.20 grams of glucose (c6h12o6) in 355 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 24.06.2019 01:30
The potential energy diagram shows the gain and loss of potential energy as water molecules decompose into hydrogen and oxygen. label the parts of the diagram.
Answers: 3
You know the right answer?
When 6.040 grams of a hydrocarbon, CxHy, were burned in a combustion analysis apparatus, 18.95 grams...
Questions

Mathematics, 12.01.2021 17:00


Mathematics, 12.01.2021 17:00


Mathematics, 12.01.2021 17:00


Business, 12.01.2021 17:00


Chemistry, 12.01.2021 17:00

Advanced Placement (AP), 12.01.2021 17:00

Social Studies, 12.01.2021 17:00

Mathematics, 12.01.2021 17:00

History, 12.01.2021 17:00






Computers and Technology, 12.01.2021 17:00

Mathematics, 12.01.2021 17:00

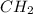 and molecular formula is
and molecular formula is 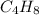
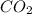 = 18.95 g
= 18.95 g
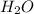 = 7.759 g
= 7.759 g
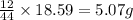 of carbon will be contained.
of carbon will be contained.
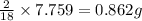 of hydrogen will be contained.
of hydrogen will be contained.