Multiple Choice, Select all that apply, and True/False
1 point
Eagles and grizzly bears live...

Chemistry, 23.12.2020 04:50 jovonjones1234
Multiple Choice, Select all that apply, and True/False
1 point
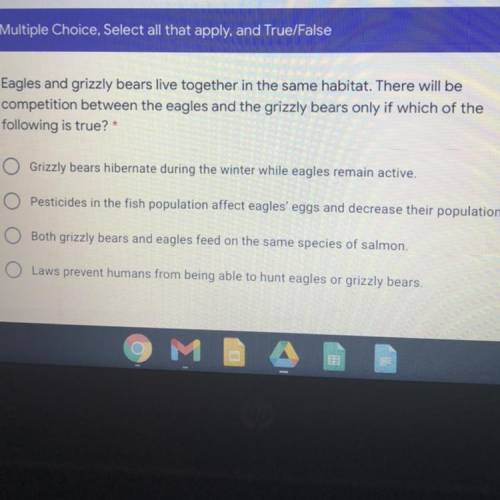
Eagles and grizzly bears live together in the same habitat. There will be
competition between the eagles and the grizzly bears only if which of the
following is true?
Grizzly bears hibernate during the winter while eagles remain active.
Pesticides in the fish population affect eagles' eggs and decrease their population.
Both grizzly bears and eagles feed on the same species of salmon.
O Laws prevent humans from being able to hunt eagles or grizzly bears.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3
You know the right answer?
Questions



History, 24.06.2019 23:00



Mathematics, 24.06.2019 23:00

Mathematics, 24.06.2019 23:00


Mathematics, 24.06.2019 23:00



Spanish, 24.06.2019 23:00

Business, 24.06.2019 23:00


Arts, 24.06.2019 23:00

Mathematics, 24.06.2019 23:00






