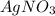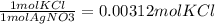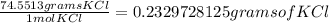Chemistry, 23.12.2020 17:40 biaxialpower789
11. Silver ions can be precipitated from aqueous solutions by the addition of aqueous chloride:
KCl(aq) + AgNO3(aq) → AgCl(s) + KNO3(aq)
Silver chloride is virtually insoluble in water so that the reaction appears to go to completion. How many grams of solid KCl must be added to 25.0 mL of 0.125 M AgNO3 solution to completely precipitate the silver?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2


Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3
You know the right answer?
11. Silver ions can be precipitated from aqueous solutions by the addition of aqueous chloride:
KCl...
Questions

Mathematics, 11.03.2021 23:00

Social Studies, 11.03.2021 23:00



Mathematics, 11.03.2021 23:00

History, 11.03.2021 23:00

History, 11.03.2021 23:00

Chemistry, 11.03.2021 23:00



English, 11.03.2021 23:00


Physics, 11.03.2021 23:00

Mathematics, 11.03.2021 23:00

English, 11.03.2021 23:00

Spanish, 11.03.2021 23:00

Mathematics, 11.03.2021 23:00

Mathematics, 11.03.2021 23:00


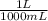 ) = 0.025 L
) = 0.025 L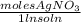 ) = 0.003125 moles
) = 0.003125 moles