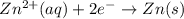Chemistry, 24.12.2020 02:10 ciaralamont2
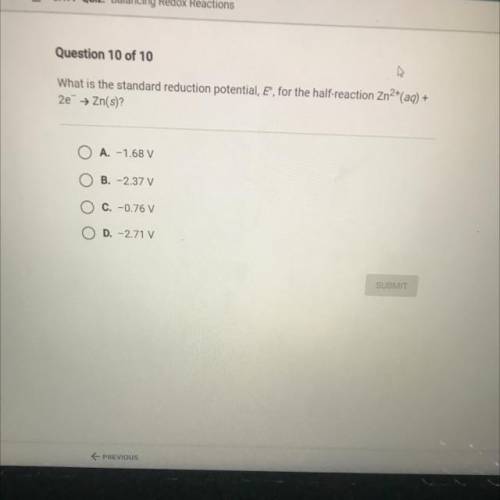
What is the standard reduction potential, E, for the half-reaction Zn2+(aq)+2e- -> Zn(s)?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:30
What is the molecular formula of a hydrocarbon with m+ = 166? (write the formula with no subscripts, e.g. c4h10.) what is the sum of rings and double bonds in this compound?
Answers: 1

Chemistry, 21.06.2019 19:00
Asyringe contains 56.05 ml of gas at 315.1 k. what volume will that gas occupy if the temperature is increased to 380.5 k? a) 12.41 b) 46.42 c) 67.68 d) 81.74
Answers: 1

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
What is the standard reduction potential, E, for the half-reaction Zn2+(aq)+2e- -> Zn(s)?
...
...
Questions

Mathematics, 27.10.2020 17:00


Mathematics, 27.10.2020 17:00



Mathematics, 27.10.2020 17:00


Geography, 27.10.2020 17:00

Social Studies, 27.10.2020 17:00


World Languages, 27.10.2020 17:00

English, 27.10.2020 17:00

Social Studies, 27.10.2020 17:00

SAT, 27.10.2020 17:00

English, 27.10.2020 17:00




Mathematics, 27.10.2020 17:00