
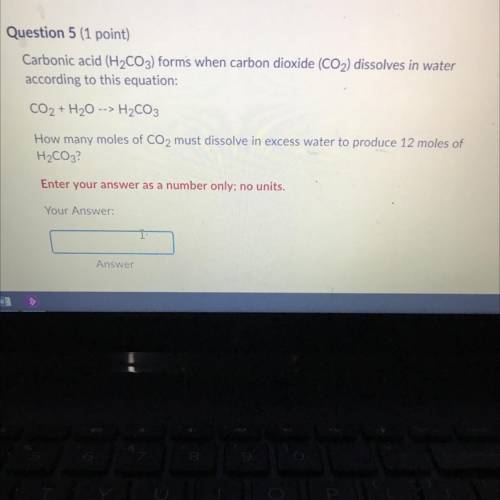
Chemistry, 24.12.2020 03:20 kenzielema12
Carbonic acid (H2CO3) forms when carbon dioxide (CO2) dissolves in water
according to this equation:
CO2 + H2O
H2CO3
How many moles of CO2 must dissolve in excess water to produce 12 moles of
H2CO3?
Enter your answer as a number only; no units.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

You know the right answer?
Carbonic acid (H2CO3) forms when carbon dioxide (CO2) dissolves in water
according to this equation...
Questions

Arts, 06.05.2020 04:57

Mathematics, 06.05.2020 04:57




English, 06.05.2020 04:57


Mathematics, 06.05.2020 04:57



Mathematics, 06.05.2020 04:57

French, 06.05.2020 04:57



Mathematics, 06.05.2020 04:57

Mathematics, 06.05.2020 04:57

Social Studies, 06.05.2020 04:57

Mathematics, 06.05.2020 04:57

History, 06.05.2020 04:57

English, 06.05.2020 04:57



