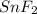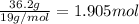Chemistry, 05.10.2019 16:20 devinluck100
Tin reacts with fluorine to form two different compounds, a and b. compound a contains 38.5 g of tin for each 12.3 g of fluorine. compound b contains 56.5 g of tin for each 36.2 g of fluorine. what is the lowest whole-number mass ratio of tin that combines with a given mass of fluorine?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
Tin reacts with fluorine to form two different compounds, a and b. compound a contains 38.5 g of tin...
Questions

Mathematics, 05.02.2020 00:01





Social Studies, 05.02.2020 00:01

Mathematics, 05.02.2020 00:01

Mathematics, 05.02.2020 00:01

English, 05.02.2020 00:01


English, 05.02.2020 00:01

Mathematics, 05.02.2020 00:01




English, 05.02.2020 00:01

History, 05.02.2020 00:01


Physics, 05.02.2020 00:01

English, 05.02.2020 00:01