Review Toples
References
Use the References to access important value in needed for its quest...

Chemistry, 25.12.2020 01:00 alazayjaime1423
Review Toples
References
Use the References to access important value in needed for its question
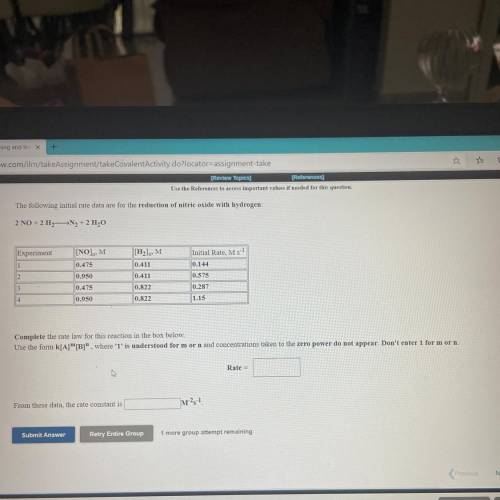
The following initial rate data are for the reduction of nitric oxide with hydrogen
2 NO 211N, 211,0
Experiment
1
INO).M
0,475
IM
0.411
mit Rate, M.
0.144
0.575
0.000
0.478
0.000
0,822
0.822
0.217
1.IS
1
Complete the rate law for this reaction in the box below
Use the form KIA"", where 'T' is understood for morn and concentrations taken to the zero power do not appear. Don't enter for morn
Rate
From these data, the rate constant is
m?!
Submit Answer
Retry Entre Group
1 more group attempt remaining

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1
You know the right answer?
Questions


Geography, 21.07.2019 23:00


Geography, 21.07.2019 23:00


Geography, 21.07.2019 23:00

History, 21.07.2019 23:00

Geography, 21.07.2019 23:00

Biology, 21.07.2019 23:00


Social Studies, 21.07.2019 23:00

Geography, 21.07.2019 23:00

History, 21.07.2019 23:00


Computers and Technology, 21.07.2019 23:00

Geography, 21.07.2019 23:00


Geography, 21.07.2019 23:00


Biology, 21.07.2019 23:00



