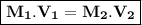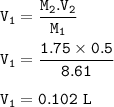Chemistry, 26.12.2020 18:00 PuppyLover3479
Describe how you would prepare 5.00 × 102 mL of a 1.75 M H2SO4 solution, starting with an 8.61 M stock solution of H2SO4

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2

Chemistry, 23.06.2019 07:40
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1
You know the right answer?
Describe how you would prepare 5.00 × 102 mL of a 1.75 M H2SO4 solution, starting with an 8.61 M sto...
Questions

History, 01.04.2021 04:10


Business, 01.04.2021 04:10



Mathematics, 01.04.2021 04:10



Biology, 01.04.2021 04:10

Mathematics, 01.04.2021 04:10


Mathematics, 01.04.2021 04:10

Mathematics, 01.04.2021 04:10

Mathematics, 01.04.2021 04:10

English, 01.04.2021 04:10



Mathematics, 01.04.2021 04:10

Mathematics, 01.04.2021 04:10