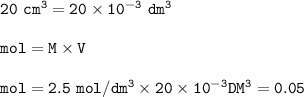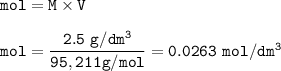Chemistry, 27.12.2020 03:00 sunarafet3814
1. Magnesium chloride solution reacts with silver nitrate solution to form magnesium nitrate
solution and silver chloride.
Equation: MgCl2 (s) + 2 AgNO3 (aq) → Mg(NO3)2 (aq) + 2 AgCl (s)
Find the mass of silver chloride formed if
(a) 20 cm of 2.5 mol/dm^3 of magnesium chloride solution is used.
(6) 20 cm of 2.5 g/dm^3 of magnesium chloride solution is used.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3


Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2
You know the right answer?
1. Magnesium chloride solution reacts with silver nitrate solution to form magnesium nitrate
soluti...
Questions

History, 05.05.2020 08:17








Chemistry, 05.05.2020 08:17

History, 05.05.2020 08:17


Mathematics, 05.05.2020 08:17

Mathematics, 05.05.2020 08:17




Mathematics, 05.05.2020 08:17


Social Studies, 05.05.2020 08:17

Social Studies, 05.05.2020 08:17