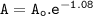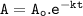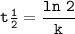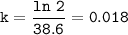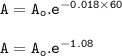Chemistry, 27.12.2020 14:30 amayareyes101
6. At 180 °C, the decomposition of a gaseous compound XO2 is a first order reaction
with the half-life 38.6 min. The initial pressure of XO2 is 372.5 kPa.
a) What is the rate of decomposition of XO2 after 1 hour?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1



Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3
You know the right answer?
6. At 180 °C, the decomposition of a gaseous compound XO2 is a first order reaction
with the half-l...
Questions

English, 05.05.2020 10:13

Mathematics, 05.05.2020 10:13


Computers and Technology, 05.05.2020 10:13


Mathematics, 05.05.2020 10:13

Mathematics, 05.05.2020 10:13

Mathematics, 05.05.2020 10:13







History, 05.05.2020 10:13

History, 05.05.2020 10:13

Mathematics, 05.05.2020 10:13



Mathematics, 05.05.2020 10:13